A shot of bacteria might seem unwanted in a clinic, but new research shows that it could be just the boost immune cells need.
A study recently published in Nature shows that bacteria can produce metabolites that make tumors better immunotherapy targets.1 Bacteria engineered to produce the T cell-enhancing amino acid L-arginine drew more T cells into tumors and bolstered the tumor-clearing response to checkpoint blockade, a form of immunotherapy that interferes with built-in controls so that a patient’s immune cells attack tumors without restrictions.
“These arginine bacteria are not going to solve all the problems, but they clearly help enhance the antitumor T cell response,” said Roger Geiger, an immunologist at Università della Svizzera italiana and senior author of the study. In the future, he added, these metabolite-producing bacteria might be part of “a catalog of strains that you need to choose from.”
Feeding an Army
T cells are one of the main foot soldiers conscripted by immunotherapy. These cells march around the body on alert for anything that might look out of the ordinary, such as a virus or a warped cancer cell, ready to multiply and eliminate their target. But tumors sometimes have their own defenses; not only do they look similar to more familiar cells, they can also inactivate T cells and evade their attacks.
In one of Geiger’s previous studies, he found that arginine was a superfood of sorts for T cells.2 It made them more resilient and more potent against tumors. So, if there was more arginine in a tumor, it might be able to amp up an army of T cells. But delivering arginine to a tumor was no simple task. One-time injections quickly dissipated. Geiger did the math and realized that to increase arginine to the level that would impact T cells, a 165-pound person would have to consume 150 grams of arginine in their diet every day. “It was quite obvious to us that this was not going to work,” said Geiger.
That’s when Geiger, whose background is in tumor immunology, learned something interesting from Jose Lora, then a vice president of research at a bacterial engineering biotechnology company called Synlogic: bacteria can preferentially colonize tumors. Synlogic was already taking advantage of this to develop a bacteria-based therapy to trigger an antitumor response from the immune cells that eat up bacteria.3 But many bacterial strains can also be genetically engineered into factories producing a specific product, such as checkpoint-blockade nanobodies made in tumors.4 By engineering bacteria to produce arginine, they might be able to create an arginine-dense environment in the tumor to feed T cells.
Calling in the Troops
Geiger teamed up with the Synlogic team to engineer an E. coli strain that doesn’t make people sick, called E. coli Nissle. Bacteria are particularly well suited to prod the immune system because, even without engineering, they have molecules on their surface that activate the immune system, said David Hava, chief scientific officer of Synlogic.
The researchers inserted a mutated version of a gene that continuously converts ammonia into arginine. Ravid Straussman, a tumor microbiome researcher at the Weizmann Institute of Science, said in an email that this approach “is very elegant as it uses relatively simple bacterial engineering… to cleverly manipulate the tumor immune landscape.”
From a manufacturing standpoint, Hava agreed that simplicity is key. Making large quantities of bacteria that are safe for humans and keeping the microbes healthy and viable so that they produce the intended metabolite in patients can be tricky. “We can do lots of engineering and lots of manipulations and lots of changes, but the simpler the better,” Hava said. “We ultimately have to kind of make and manufacture these streams for human use.”
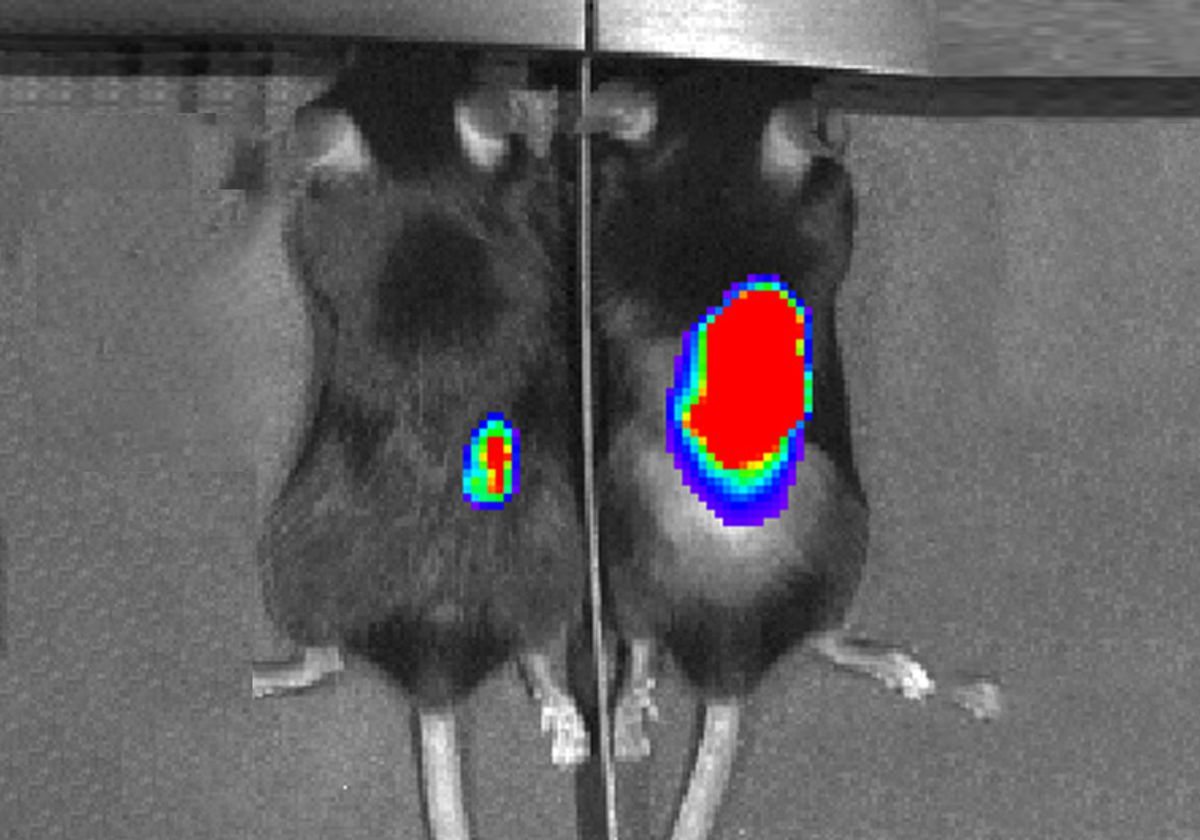
When they injected these bacteria into colon tumors in a mouse model, the bacteria quickly settled into the tumors and produced arginine, attracting an influx of T cells. However, these T cells still didn’t attack the tumors; that is where checkpoint blockade therapy came into play. When the researchers also gave the mice anti-PD-L1 antibodies to block the tumor’s T cell-inactivating defenses, three-quarters of the mice were able to successfully eradicate their tumors. Only half as many mice responded similarly to checkpoint blockade alone. These therapies are dependent on having a strong immune response to the tumors—specifically, involving T cells—and did not work well in models with few immune cells.
However, not all tumors are easily accessible for injections, Geiger said. His team also tried delivering the bacteria through injections into the blood. The bacteria were still able to find their way to larger tumors, which they could partially shrink, but missed smaller tumors.
“Initially, you find the bacteria in many organs, but after two or three days, they're completely cleared out and only colonized tumors,” Geiger said. “I find that fascinating and I'm hopeful that we can use these bacteria in the future to reach inaccessible tumors.”
The Battle Is Not Over
The bacteria-based therapy has only been tested in mouse models so far, and human patients might present more complications, Straussman said. “Patient-specific differences in the number of bacteria that reach the tumor and in the pre-treatment tumor immune landscape may impact the effectiveness of the suggested treatment,” said Straussman.
Geiger agrees that further studies are necessary to figure out what might make the bacteria more or less likely to colonize a tumor. One factor might be the microbiome, the bacteria already residing in the tumor, or other immune factors. Bacterial therapies that are currently in Synlogic’s pipeline for other diseases are starting to generate clinical data on how humans respond and how to safely deliver bacteria. For example, Synlogic’s clinical programs use bacteria that are engineered not to take up permanent residence and grow in the patient, so patients need to receive regular doses.
This system may extend to other metabolites, such as tryptophan, also known to impact T cell function. Geiger’s next steps are to see if they can engineer bacteria to make other metabolites that work in conjunction with arginine or checkpoint inhibitors. By better understanding the system and ensuring that it is safe for humans, Geiger hopes that these bacteria could one day be an “off-the-shelf” therapy for patients, especially for tumors that are good candidates for immunotherapy, such as melanoma and lung cancer.
“My dream is that we could profile a tumor and see whether there is not enough arginine, maybe there's too much adenosine, or something wrong with tryptophan,” Geiger said. “There would be a variety of [bacterial] strains available and then we could analyze the tumor and see what we need to bring into that tumor in order to make it go away.”
References
- F.P. Canale et al., “Metabolic modulation of tumours with engineered bacteria for immunotherapy,” Nature, 598(7882):662–66, 2021.
- R. Geiger et al., “L-Arginine modulates T cell metabolism and enhances survival and anti-tumor activity,” Cell, 167(3):829-842.e13, 2016.
- D.S. Leventhal et al., “Immunotherapy with engineered bacteria by targeting the STING pathway for anti-tumor immunity,” Nat Commun, 11(1):2739, 2020.
- C.R. Gurbatri et al., “Engineered probiotics for local tumor delivery of checkpoint blockade nanobodies,” Sci Trans Med, 12(530):eaax0876, 2020.